Abstract
Background
Age has long been recognized as a poor prognostic factor in diffuse large B-cell lymphoma (DLBCL) and more recently, a lower baseline quality of life (QOL) in patients with aggressive lymphomas has been associated with an inferior overall survival (OS) (Thompson et al, 2018). Older patients with DLBCL are a heterogeneous population and commonly have comorbid conditions. A concerted effort to identify prognostic factors specific to this population is needed, including QOL. Event free survival at 24 months (EFS24) is a well validated endpoint in DLBCL (Maurer et al, 2014) and is clinically relevant in older patients. We hypothesized that baseline clinical factors and QOL would be associated with EFS24 this patient group.
Methods
Newly diagnosed lymphoma patients were prospectively enrolled within 9 months of diagnosis into the University of Iowa/Mayo Clinic Lymphoma SPORE Molecular Epidemiology Resource (MER). Patients ≥70 years of age with DLBCL were utilized in this study. All pathology was reviewed by a lymphoma hematopathologist. Clinical variables were abstracted from the medical record. QOL was assessed at enrollment using a single item Linear Analogue Self-Assessment (LASA) and the 27-item Functional Assessment of Cancer Treatment-General questionnaire (FACT-G), which includes physical, social, emotional, and functional well-being domains. QOL scores were converted to a 0-100 scale to facilitate modeling. QOL was analyzed in patients who completed 80% of the FACT-G questions. Characteristics of the study population were compared using the Wilcoxon rank sum test. Odds ratios (ORs) and 95% confidence intervals (CI) were used to estimate the associations of clinical and continuous QOL variables with failure to achieve EFS24 using logistic regression models, with a stepwise approach to identify parsimonious multivariate models.
Results
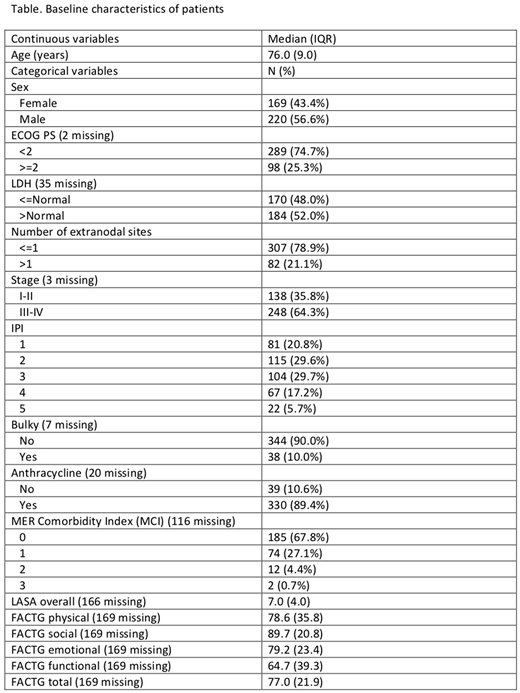
A total of 389 patients ≥70 years were identified with DLBCL in the MER from 2002-2015; patient characteristics are described in the Table. In the entire study population, univariate predictors of EFS24 failure included age (OR per 10 years=1.85, 95% CI: 1.26-2.71), ECOG PS ≥2 (OR=4.78, 95% CI: 2.92-7.83), elevated LDH (OR=3.59, 95% CI: 2.27-5.65), stage III-IV (OR=1.94, 1.25-3.02), bulky disease (OR=2.86, 95% CI: 1.43-5.72), international prognostic index (OR=8.71 for IPI 5 vs IPI 0, 95% CI: 3.04-24.9), and receipt of an anthracycline (OR=0.25, 95% CI: 0.12-0.51), while none of the other variables in the Table, including the QOL variables, were statistically significant. In a stepwise multivariate analysis, only ECOG PS ≥2 (OR= 3.56, 95% CI: 2.09-6.06) and elevated LDH (OR=2.86, 95% CI: 1.78-4.60) remained significant predictors of EFS24 failure. QOL data were available on 220 patients, with 120 completing QOL questionnaires prior to receipt of chemotherapy. Compared to participants who completed their FACT-G before the initiation of chemotherapy, the median FACT-G physical (85.7 v. 71.4, p=0.005) and functional (71.4 vs 57.1, p=0.008) scores were lower in participants completing FACT-G after initiation of therapy, were higher on FACT-G emotional (79.2 vs 83.3, p=0.03), and showed no differences on FACT-G social (89.3 vs 91.7, p=0.60) or FACT-G total score (78.2 vs 74.1, p=0.17). Given these differences, we restricted the QOL analysis to those with pre-treatment data. In univariate analysis, the LASA score (OR= 0.98, 95% CI: 0.84-1.14), the FACT-G total (OR=0.99, 95% CI: 0.96-1.01), physical (OR=0.98, 95% CI: 0.97-1.00), social (OR=1.00, 95% CI: 0.98-1.03), emotional (OR= 1.00, 95% CI: 0.98-1.02), and functional (OR=0.99, 95% CI: 0.98-1.01) scores were not associated with EFS24 failure. In a stepwise multivariate analysis of clinical variables in patients with pretreatment QOL scores, only ECOG PS ≥2 (OR=3.56, 95% CI: 1.30-9.72) and female sex (OR=0.37, 95% CI: 0.16-0.85) were associated with failure to achieve EFS24.
Conclusions
In patients aged 70 years and older, ECOG PS and LDH were the strongest independent predictors of failure to achieve EFS24. As expected, physical and functional scores were lower in patients who completed their QOL after initiation of treatment. Restricting the analysis to patients with pre-treatment data, QOL was not associated with failure to achieve EFS24. Additional studies are needed to identify the most robust clinical predictors of EFS24, while QOL does not appear to be an important predictor of EFS24.
Reagan:Seattle Genetics: Research Funding. Maurer:Morphosys: Research Funding; Celgene: Research Funding; Nanostring: Research Funding. Cerhan:Celgene: Research Funding; Nanostring: Research Funding; Jannsen: Other: Scientific Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal